Half century of peptide synthesis
Wojciech Kamysz
R&D laboratory Lipopharm.pl

In these times, high hopes of applications are associated with biopolymers such as peptides and proteins. This fascination lasts more than fifty years. In 1954 Vigneaud described the synthesis of oxitocin. As a result, theories describing the replacement of conventional medicines by synthetic peptides began to appear. Nowadays, annual production of peptide drugs (excluding insulin) is about one tonne. Insulin is mainly manufactured by two potentates – Novo Nordisc (Denmark) and Eli Lilly (USA) – and annual production is around 20 tonnes. In this connection the market of peptide drugs with exception of insulin, is very poor. This happens mainly because of the problems faced by producers among which the most important is the high cost of the peptides preparation. Moreover, there are significant problems with formulation (low bioavailability, biological and chemical instability), and with the storage of peptide drugs. Isolation of the product (drug or drug form) from unfavourable conditions for example by appropriate packaging, coating the drug form (whole or fragments) using as a micro-compartment (liposomes, micro- and nanocapsules), the use of cyclodextrins, or a change in the degree of fragmentation does not always solves the problem. Sometimes the most effective is application of auxiliary substances guaranteeing stability, enzyme inhibitors (e.g. protease inhibitors), antioxidants (ascorbic acid, sodium ascorbate, glutathione, sodium thiosulphate) or modification of chemical structure (the use of D- amino acids instead of L).
Solid phase peptide synthesis
Solid-phase peptide synthesis (SPPS) was invented over 50 years ago. The commonly known approach (called after the name of the inventor), the Merrifield method, has many advantages. Thanks to this technique the synthesis of peptides is relatively rapid nowadays and is often performed by using machines known as peptide synthesizers. First devices of this kind were created in the 1960’s. The SPPS technique involves attachment of the substrate to an insoluble inorganic phase (a polymer) that connects to a subsequent segments – amino acids. Beforehand, the carrier is specially prepared in order to obtain a suitable linker to which the first amino acid is attached. The linker is a chemical moiety which is linking the insoluble polymer to the first amino acid. This ensures stable connection during the synthesis and easy peptide cleavage during deprotection of the final product. The synthesis on the solid phase provides a great deal of advantages. Over recent years it has grown rapidly folowing the synthesis of various organic molecules from biopolymers such as nucleic acids, oligo- and polysaccharides to low molecular weight compounds. The excess of unreacted feedstock is easily washed away in specialized vessels commonly called Merrifield’s vessels. This approach allows the use of a large excess of reagents to minimize the scale of synthesis and the use of toxic substrates. In the last five(ten)-years, the previously mentioned ability to automate the SPPS has been enriched in the possibility of the synthesis by a fully automated microwave synthesizer, so that the coupling process of amino acids can be accelerated up to 10 times.
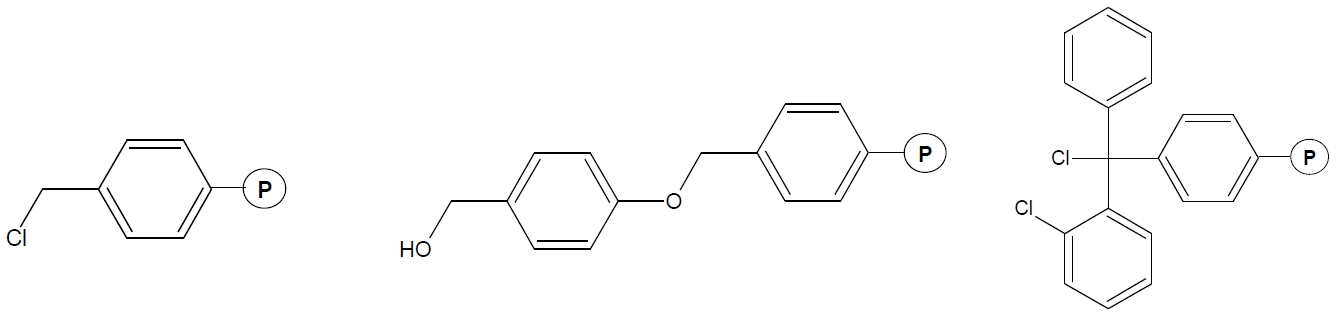
Fig. 1. Examples of carriers (resin) for the synthesis of peptides. A - Merrifield, B - Wang, C - 2-chlorotrityl
Boc, Fmoc chemistry and nothing more…
Chemical synthesis of peptides is based on the use of protecting groups. The key point in the design of the synthesis procedure is the use of the orthogonality of the side chains covers of amino acids and the α-amino group protection of each of the connected substrate. Since the days of pioneering discoveries based on the solid-supported synthesis, the tert-Butyloxycarbonyl group (Boc) is used to protect the amino groups. The Boc moiety is usually cleaved by using trifluoroacetic acid and does not belong to willingly used protecting groups in the step by step synthesis. In addition, Boc strategy requires liquid hydrogen fluoride to cleave the peptide from the carrier. Simultaneously in the 1970s was introduced a Fmoc protecting group (9-fluorenylmethoxycarbonyl), which is cleaved by piperidine. Owing the low cost of the Fmoc amino acid derivatives, it has become common in laboratories 20 years later. In both synthetic approaches the same condensing and anti-racemization agents are used (the same for more than 50 years!). Despite the fact that the new carriers are emerging and catalogs dedicated for peptide laboratories are full of novelties, most of them are based on the same linkers. For marketing reasons, extraordinary properties are being attributed to carriers. In fact, they are usually polymers based on polystyrene crosslinked with divinylbenzene in an amount of 0.5-2%. It is estimated that resins based on polystyrene make up 90% of all carriers used in the organic synthesis. In practice, the choice of the resin used in the synthesis of the peptides depends on the structure of the synthesised peptide (e.g. free carboxyl or amido group at the C-terminal amino acid), and whether the peptides are short or long. In the case of the synthesis of longer peptides, carriers with less deposition of functional groups are used.
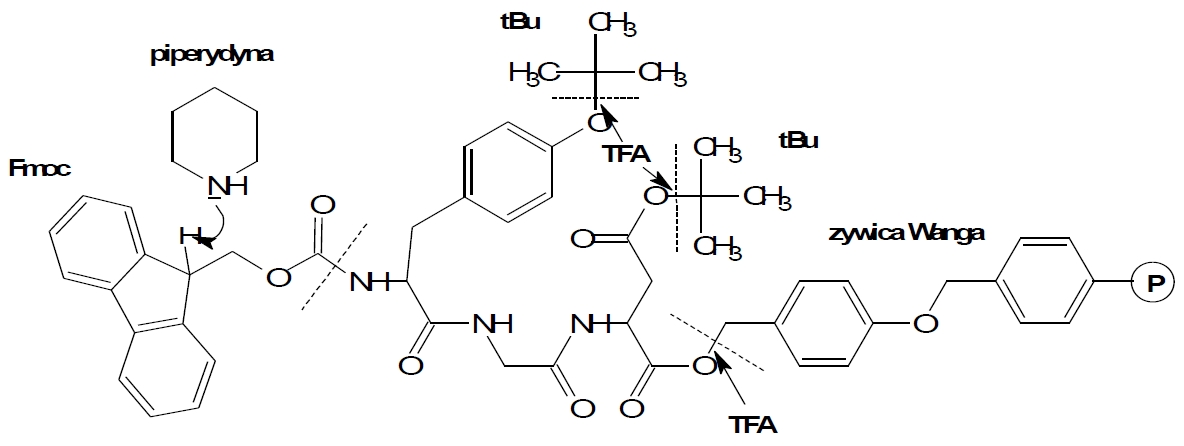
Fig. 2. The reaction scheme of synthesis using Fmoc chemistry.
Purification and analysis
Purification of the peptides belongs to the activities of high difficulty. The impurities remaining in the sample are often peptides with a similar structure. The purification process is often complicated by the possibility of chemical changes during the sample preparation or chromatographic separation. An example is the oxidation of free cysteine residues to disulphide bonds, the N-terminal cyclization of glutamine to pyroglutamic acid residues or a modification of amino acids by attaching reactive derivatives covers during deprotection of resin-bound peptides. Classic column chromatography on silica gel was the first method for purification of peptides. It was soon superseded by gel filtration on Sephadex beads and ion exchange chromatography. Until the 1980s, these techniques were the only proficient methods for purification of synthetic peptides. Since the introduction of high-performance liquid chromatography (HPLC) they are now only of historical interest and the young generation of researchers usually are not even able to use them. Reverse-phase HPLC requires expensive equipment, so that at the present time not all research teams are capable of independent synthesis and purification of the peptides. The simplest preparative chromatograph with a column costs about 100 thousand PLN. The costs of the eluents, materials and maintenance costs of equipment which are prone to crashes should also be taken into account. The dry sample is obtained by lyophylization. A further constraint is the identification of the resulting products. Both the performance of the amino acid analysis (currently one departs from it) as well as obtaining a mass spectrum requires a sophisticated equipment or cooperation concerning expensive analysis held by specialised laboratories. Sometimes it happens that several products are obtained after the synthesis and these costs are even higher. Mass spectrometry coupled with matrix-assisted laser desorption/ionization and time of flight analyzer (MALDI-TOF) gives the most benefits nowadays. The most modern mass spectrometers are also able to determine the amino acid sequence and the structure of contaminants.
Combinatorial chemistry and unfulfilled hopes
The possibility of coupling amino acids in various combinations in conjunction with polymer-supported synthesis resulted in the development of parallel and combinatorial chemistry. One of the examples is a: Geysen procedure developed in 1984 (amino acid deposition on polyethylene rods, which are immersed in the reaction vessels with the respective amino acid), and a year later published the Houghten's tea bags method (resin placed in a perforated polypropylene bags). Important milestone could turn out to be published in 1988 by Arpad Furka a portioning-mixing method, which consists in moving from the control over the sequence of synthesised peptides and requires only sequential dividing of solid support resin and mixing with the peptides. However, considering the number of scientific reports related to combinatorial chemistry, the actual effects are not too impressive.


